9 Nov
5 Things You Need To Know About Paragard IUDs
Florida Law Group Defective Medical Devices
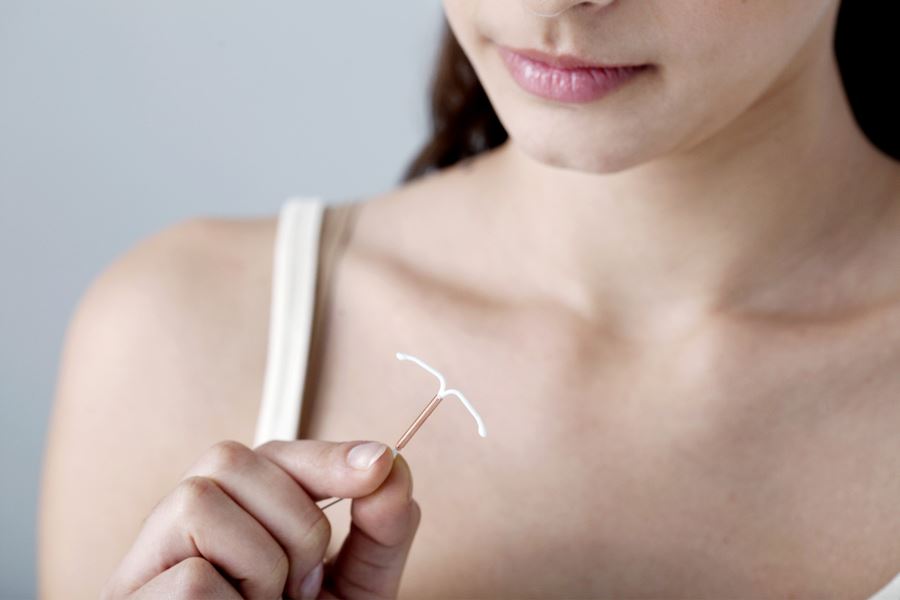
What are Paragard IUDs?
Paragard IUDs are a small, completely hormone-free form of contraception. They are T-shaped, made of flexible plastic wrapped in a thin layer of copper, and inserted/removed non surgically during routine office visits by healthcare providers. Paragard IUDs are used to prevent pregnancy for up to 10 years (if desired) before they need to be replaced.
Why are lawyers talking about Paragard IUDs?
While Paragard IUDs have been around for over three decades, they are gaining media and legal attention for many lawsuits that have been filed because of claims that the manufacturer, Teva, did not adequately warn users or healthcare providers about the possibility of device breakage and misrepresented the device as safe when it was actually defective. Many women have experienced devastating injuries because the Paragard IUDs broke apart during removal or otherwise malfunctioned.
According to the CDC, 10.3% of women aged 15-49 are currently using intrauterine devices like the Paragard IUD to prevent pregnancy. If you or a loved one has experienced an injury or adverse effects due to using the Paragard IUD, or if you are considering the Paragard IUD as your form of birth control, here are 5 important things you should know about Paragard IUDS:
- The Paragard IUD is FDA approved, but there are many reports of device breakage.
- The device is marketed as “safe” and “effective”.
- The Paragard IUD is the only copper IUD available in the United States.
- Paragard IUDs can have dangerous side effects.
- Currently, the device is making headlines due to numerous personal injury lawsuits against the manufacturers.
Paragard IUDs are the only intrauterine devices that have been approved by the Food and Drug Administration for over 30 years. First approved by the FDA in 1984, the original manufacturer (Teva Pharmaceuticals, which sold the rights to CooperSurgical, Inc. in 2017) began marketing the device in 1988.
However, just because something is FDA approved does not mean that it is risk-free; it only means that the agency determined that the benefits of the product outweigh the known risks for intended use. Their approval process, which is not the same for all products and not as stringent as many consumers would like to believe, is based on data supplied by the manufacturers themselves, which means that the information may be biased or purposely misleading in order to gain FDA approval. Though it seems impressive that the Paragard IUD has kept FDA approval for so long, that distinction is not synonymous with safety.
In the case of the Paragard IUD, the device has not yet been recalled, but the FDA has received over 1,600 reports of breakage since 2010.
If you visit Paragard IUD’s website, you will see many exciting, attractive claims that they make about their product. They say it’s 100% hormone-free and over 99% effective. They say that it is a “no-hassle, low maintenance” form of birth control. They say it is “immediately reversible whenever you decide”. They say it is the longest lasting prescription birth control method available, providing continuous pregnancy prevention for up to 10 years. They say it has been “clinically proven safe and effective” for over 30 years.
However, evidence from the cases of a growing number of women who are coming forth with horror stories of injuries resulting from the Paragard IUD suggests that these claims may be misleading, and that the manufacturer is withholding important information about the very real safety concerns that exist.
One of the reasons that the Paragard IUD is a popular choice for women is because of the technology of the device. The Paragard IUD is the only copper IUD. It works by continuously releasing one ingredient – copper – into the uterus. The copper acts as a strong spermicide, preventing the sperm from reaching the egg and also changing the lining of the uterus to reduce the risk of implantation.
Though the copper is effective, it is more likely to result in the expulsion of the device, which can be extremely dangerous and lead to injuries. One study revealed that 10.2% of women using Paragard IUDs experienced either expulsion or the device migrating to other places, while just 4.9% of Mirena (a hormonal IUD) users did. The copper also has been found to be fractured or missing completely upon removal, or to be embedded in the uterus, in several cases.
Some of the side effects associated with the Paragard IUD include: anemia, backaches, dysmenorrhea, dyspareunia, leukorrhea, prolonged menstrual flow, menstrual spotting, pain and cramping, urticarial allergic skin reaction, vaginal discharge, and vaginitis. A link has also been associated with Paragard IUDs and a condition called Pseudotumor Cerebri (PTC), in which the symptoms of a brain tumor are exhibited but no brain tumor actually exists. This can lead to vomiting, impaired vision, dizziness, back pain, and more.
Those are the less serious and perhaps less common side effects. Severe complications, and the reasons for many of the lawsuits that are currently being brought against the Paragard IUD manufacturer, include IUD breakage or fracture upon removal, the IUD embedding in the uterus, IUD migration or expulsion, ectopic pregnancy (outside the uterus), pelvic infections, perforation of the uterine wall or cervix, scarring and organ damage, and even death. Complications caused by the Paragard IUD can lead to the need for surgeries, including hysterectomies, which can be heartbreaking to couples who wished to conceive.
Because the Paragard IUD has left many women injured, the victims have begun to file lawsuits seeking compensation for their injuries. These lawsuits are happening in many different states. In Florida, where The Florida Law Group offers representation, one woman’s device migrated out of her uterus, becoming embedded in her colon and requiring surgery to remove the device. In 2015, independent researchers looked at 7 cases where women experienced injuries due to Paragard IUDS. The women were between the age of 28-48, and their complications included device breakage during removal or embedded copper. 6 out of the 7 women required hysteroscopic (surgical) removal of the IUD.
If you were injured by a Paragard IUD, you have the legal right to seek compensation.
Being injured by a contraceptive can take a toll emotionally, physically, and financially. If you suffered complications from the Paragard IUD that required surgery or otherwise interfered with your health, you may be able to recover monetary compensation for your injuries. The Florida Law Group’s Paragard IUD lawyers can help you understand your legal options and make a strong claim. We have recovered over a billion dollars for our clients! Call today to schedule a free consultation and get started with us. You don’t have to pay us unless we recover damages for you!
What are Paragard IUDs?
Paragard IUDs are a small, completely hormone-free form of contraception. They are T-shaped, made of flexible plastic wrapped in a thin layer of copper, and inserted/removed non surgically during routine office visits by healthcare providers. Paragard IUDs are used to prevent pregnancy for up to 10 years (if desired) before they need to be replaced.
Why are lawyers talking about Paragard IUDs?
While Paragard IUDs have been around for over three decades, they are gaining media and legal attention for many lawsuits that have been filed because of claims that the manufacturer, Teva, did not adequately warn users or healthcare providers about the possibility of device breakage and misrepresented the device as safe when it was actually defective. Many women have experienced devastating injuries because the Paragard IUDs broke apart during removal or otherwise malfunctioned.
According to the CDC, 10.3% of women aged 15-49 are currently using intrauterine devices like the Paragard IUD to prevent pregnancy. If you or a loved one has experienced an injury or adverse effects due to using the Paragard IUD, or if you are considering the Paragard IUD as your form of birth control, here are 5 important things you should know about Paragard IUDS:
- The Paragard IUD is FDA approved, but there are many reports of device breakage.
- The device is marketed as “safe” and “effective”.
- The Paragard IUD is the only copper IUD available in the United States.
- Paragard IUDs can have dangerous side effects.
- Currently, the device is making headlines due to numerous personal injury lawsuits against the manufacturers.
Paragard IUDs are the only intrauterine devices that have been approved by the Food and Drug Administration for over 30 years. First approved by the FDA in 1984, the original manufacturer (Teva Pharmaceuticals, which sold the rights to CooperSurgical, Inc. in 2017) began marketing the device in 1988.
However, just because something is FDA approved does not mean that it is risk-free; it only means that the agency determined that the benefits of the product outweigh the known risks for intended use. Their approval process, which is not the same for all products and not as stringent as many consumers would like to believe, is based on data supplied by the manufacturers themselves, which means that the information may be biased or purposely misleading in order to gain FDA approval. Though it seems impressive that the Paragard IUD has kept FDA approval for so long, that distinction is not synonymous with safety.
In the case of the Paragard IUD, the device has not yet been recalled, but the FDA has received over 1,600 reports of breakage since 2010.
If you visit Paragard IUD’s website, you will see many exciting, attractive claims that they make about their product. They say it’s 100% hormone-free and over 99% effective. They say that it is a “no-hassle, low maintenance” form of birth control. They say it is “immediately reversible whenever you decide”. They say it is the longest lasting prescription birth control method available, providing continuous pregnancy prevention for up to 10 years. They say it has been “clinically proven safe and effective” for over 30 years.
However, evidence from the cases of a growing number of women who are coming forth with horror stories of injuries resulting from the Paragard IUD suggests that these claims may be misleading, and that the manufacturer is withholding important information about the very real safety concerns that exist.
One of the reasons that the Paragard IUD is a popular choice for women is because of the technology of the device. The Paragard IUD is the only copper IUD. It works by continuously releasing one ingredient – copper – into the uterus. The copper acts as a strong spermicide, preventing the sperm from reaching the egg and also changing the lining of the uterus to reduce the risk of implantation.
Though the copper is effective, it is more likely to result in the expulsion of the device, which can be extremely dangerous and lead to injuries. One study revealed that 10.2% of women using Paragard IUDs experienced either expulsion or the device migrating to other places, while just 4.9% of Mirena (a hormonal IUD) users did. The copper also has been found to be fractured or missing completely upon removal, or to be embedded in the uterus, in several cases.
Some of the side effects associated with the Paragard IUD include: anemia, backaches, dysmenorrhea, dyspareunia, leukorrhea, prolonged menstrual flow, menstrual spotting, pain and cramping, urticarial allergic skin reaction, vaginal discharge, and vaginitis. A link has also been associated with Paragard IUDs and a condition called Pseudotumor Cerebri (PTC), in which the symptoms of a brain tumor are exhibited but no brain tumor actually exists. This can lead to vomiting, impaired vision, dizziness, back pain, and more.
Those are the less serious and perhaps less common side effects. Severe complications, and the reasons for many of the lawsuits that are currently being brought against the Paragard IUD manufacturer, include IUD breakage or fracture upon removal, the IUD embedding in the uterus, IUD migration or expulsion, ectopic pregnancy (outside the uterus), pelvic infections, perforation of the uterine wall or cervix, scarring and organ damage, and even death. Complications caused by the Paragard IUD can lead to the need for surgeries, including hysterectomies, which can be heartbreaking to couples who wished to conceive.
Because the Paragard IUD has left many women injured, the victims have begun to file lawsuits seeking compensation for their injuries. These lawsuits are happening in many different states. In Florida, where The Florida Law Group offers representation, one woman’s device migrated out of her uterus, becoming embedded in her colon and requiring surgery to remove the device. In 2015, independent researchers looked at 7 cases where women experienced injuries due to Paragard IUDS. The women were between the age of 28-48, and their complications included device breakage during removal or embedded copper. 6 out of the 7 women required hysteroscopic (surgical) removal of the IUD.
If you were injured by a Paragard IUD, you have the legal right to seek compensation.
Being injured by a contraceptive can take a toll emotionally, physically, and financially. If you suffered complications from the Paragard IUD that required surgery or otherwise interfered with your health, you may be able to recover monetary compensation for your injuries. The Florida Law Group’s Paragard IUD lawyers can help you understand your legal options and make a strong claim. We have recovered over a billion dollars for our clients! Call today to schedule a free consultation and get started with us. You don’t have to pay us unless we recover damages for you!
Paragard IUDs are the only intrauterine devices that have been approved by the Food and Drug Administration for over 30 years. First approved by the FDA in 1984, the original manufacturer (Teva Pharmaceuticals, which sold the rights to CooperSurgical, Inc. in 2017) began marketing the device in 1988.
However, just because something is FDA approved does not mean that it is risk-free; it only means that the agency determined that the benefits of the product outweigh the known risks for intended use. Their approval process, which is not the same for all products and not as stringent as many consumers would like to believe, is based on data supplied by the manufacturers themselves, which means that the information may be biased or purposely misleading in order to gain FDA approval. Though it seems impressive that the Paragard IUD has kept FDA approval for so long, that distinction is not synonymous with safety.
In the case of the Paragard IUD, the device has not yet been recalled, but the FDA has received over 1,600 reports of breakage since 2010.
If you visit Paragard IUD’s website, you will see many exciting, attractive claims that they make about their product. They say it’s 100% hormone-free and over 99% effective. They say that it is a “no-hassle, low maintenance” form of birth control. They say it is “immediately reversible whenever you decide”. They say it is the longest lasting prescription birth control method available, providing continuous pregnancy prevention for up to 10 years. They say it has been “clinically proven safe and effective” for over 30 years.
However, evidence from the cases of a growing number of women who are coming forth with horror stories of injuries resulting from the Paragard IUD suggests that these claims may be misleading, and that the manufacturer is withholding important information about the very real safety concerns that exist.
One of the reasons that the Paragard IUD is a popular choice for women is because of the technology of the device. The Paragard IUD is the only copper IUD. It works by continuously releasing one ingredient – copper – into the uterus. The copper acts as a strong spermicide, preventing the sperm from reaching the egg and also changing the lining of the uterus to reduce the risk of implantation.
Though the copper is effective, it is more likely to result in the expulsion of the device, which can be extremely dangerous and lead to injuries. One study revealed that 10.2% of women using Paragard IUDs experienced either expulsion or the device migrating to other places, while just 4.9% of Mirena (a hormonal IUD) users did. The copper also has been found to be fractured or missing completely upon removal, or to be embedded in the uterus, in several cases.
Some of the side effects associated with the Paragard IUD include: anemia, backaches, dysmenorrhea, dyspareunia, leukorrhea, prolonged menstrual flow, menstrual spotting, pain and cramping, urticarial allergic skin reaction, vaginal discharge, and vaginitis. A link has also been associated with Paragard IUDs and a condition called Pseudotumor Cerebri (PTC), in which the symptoms of a brain tumor are exhibited but no brain tumor actually exists. This can lead to vomiting, impaired vision, dizziness, back pain, and more.
Those are the less serious and perhaps less common side effects. Severe complications, and the reasons for many of the lawsuits that are currently being brought against the Paragard IUD manufacturer, include IUD breakage or fracture upon removal, the IUD embedding in the uterus, IUD migration or expulsion, ectopic pregnancy (outside the uterus), pelvic infections, perforation of the uterine wall or cervix, scarring and organ damage, and even death. Complications caused by the Paragard IUD can lead to the need for surgeries, including hysterectomies, which can be heartbreaking to couples who wished to conceive.
Because the Paragard IUD has left many women injured, the victims have begun to file lawsuits seeking compensation for their injuries. These lawsuits are happening in many different states. In Florida, where The Florida Law Group offers representation, one woman’s device migrated out of her uterus, becoming embedded in her colon and requiring surgery to remove the device. In 2015, independent researchers looked at 7 cases where women experienced injuries due to Paragard IUDS. The women were between the age of 28-48, and their complications included device breakage during removal or embedded copper. 6 out of the 7 women required hysteroscopic (surgical) removal of the IUD.

